Your support is very much appreciated. My articles and research take up a considerable amount of time and hopefully, with your support, the quality and quantity will keep increasing. Please consider taking out a paid subscription to support independent investigative journalism.
In January a federal judge in the US ordered that the Food and Drug Administration (FDA) release the data it relied on to approve Pfizer’s Covid-19 vaccine. The FDA had requested that they only release 500 pages a month, meaning the full release would have taken over 75 years! The reason given for this long timescale was that the FDA was short-staffed and only had the bandwidth to review 500 pages a month.
However, in a win for transparency, the judge ordered that the FDA needed to publish 55,000 pages a month, meaning that all of the data will be published by the end of summer 2022, rather than by 2097.
The first tranche of documents were released last night and I take an initial look at some of them below. Whilst the Ukraine crisis may be a good time to bury bad news, anything of interest should be highlighted, examined by the experts and ultimately reported on in the press.
Distribution of Lipid-nanoparticles (LNP) around the body
The LNPs are used to deliver fragile mRNA strands into cells. They are tiny balls of fat which protect the mRNA from being destroyed and allow it to enter cells undetected. Toxicity of LNPs has always been a concern but I am not expert enough to comment on whether they are or are not toxic in the Pfizer vaccine.
From early on in the vaccination programme, fact-checkers such as this one, assured us that mRNA “is likely to stay right in the arm where it’s injected and get taken up by the cells there”. We know this is not correct and these latest documents confirm that.
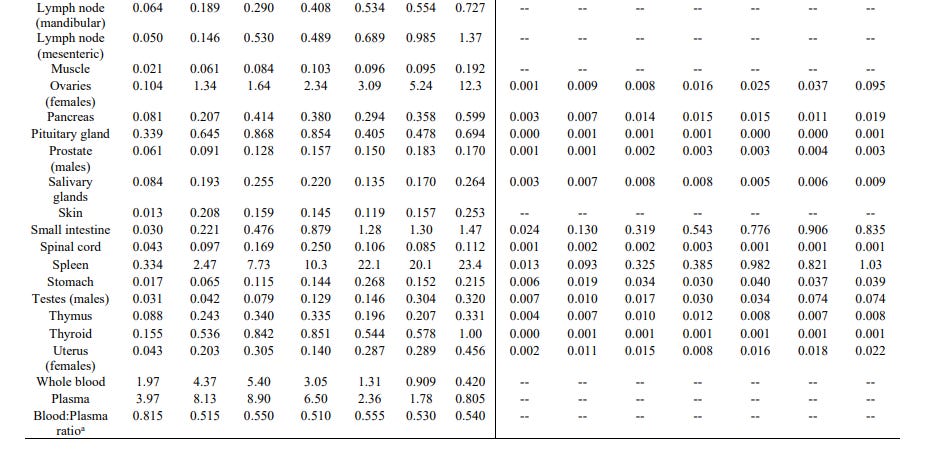
This document - a Nonclinical Overview and this Pharmacokinetics Written Summary looked at the distribution of LNPs in the blood, plasma and selected tissues of Wistar Han rats, over 48 hours after a single injection. The full results can be seen here.
As you can see, the LNPs were present in all the organs tested. As would be expected, the injection site had the highest levels which started declining after 4 hours. At 48 hours the highest concentration of LNPs were at the injection site and represented almost 25% of the administered dose.
However, there were high concentrations of LNPs in other organs, just not as high as at the injection site. The next highest was in the liver with over 16% of the administered dose in that organ at 48 hours. High concentrations were also seen in adrenal glands, spleen, bone marrow and ovaries.
What is concerning about this is that by 48 hours the concentration of LNPs was falling in the injection site but still rising in the other organs listed above. It was also still rising in many other organs where the concentration was much lower.
What happened after 48 hours? How much did the concentration of LNPs rise in the other organs and for how long? Why did the study finish at 48 hours or not get extended after it was obvious the levels were still climbing?
Earlier in the document, it says distribution to the liver is likely mediated by LNPs entering the blood stream and that there was no evidence of liver injury. It says, at the injection site, levels dropped to background levels after 9 days.
Another Final Report also looked at the distribution of LNPs following intramuscular administration in Wistar Han Rats in more detail.
Initially, 21 male rats were dosed with 100ug mRNA but some adverse clinical signs after 24 hours meant they reduced the dose to 50ug. One of the 21 rats started declining with irregular respiration and after 30 hours was piloerect, hunched and hypersensitive to noise stimulus. The animal had to be humanely killed and the diagnostic necropsy showed a finding in the liver. The levels of LNPs in this rat are not documented alongside the other results so it would be interesting to know what they were like. All of the animals in this 100ug group had a reduction in bodyweight and didn’t eat their food. No adverse effects were noted in any males in the 50ug group.
In the 50ug group of female rats, after 30 hours, one animal had irregular respiration and after 48 hours was hunched and piloerect.
Concentrations of LNPs were higher in adrenal glands and the injection site in males and higher in the liver and spleen in females.
Whilst the highest percentage of the administered dose was found in the injection site in males, it was actually highest in the liver for females.
I will continue looking through the other documents and report on them in due course so subscribe to my newsletter for further updates.





You wrote:
‘What happened after 48 hours? How much did the concentration of LNPs rise in the other organs and for how long? Why did the study finish at 48 hours or not get extended after it was obvious the levels were still climbing?’
This is classic Fauci. When things are looking bad, stop the trial before things get dire and say the evidence is actually positive, and that the nature of the emergency requires that we get this drug to market ASAP.
He did this with AIDS meds and he did this during Covid.
What is most interesting to me is how this information just did not, and still does not, cause piloerection of the FDA and VRBPAC members neck hairs. With evidence that LNP accumulates in the liver and the recent in vitro study showing reverse transcription of Pfizer mRNA into hepatocytes one must ask what else did they know about bio distribution? And what clinical impact could it have on humans both short and longterm. Thank you for this summary.