BMJ Investigation - Are Drug Regulators for Hire? Leaked emails and a Doctor explains his vaccine position in a letter to the Disciplinary Body
From the FDA to the MHRA
Your support is very much appreciated. Please consider taking out a paid subscription to support independent journalism.
The British Medical Journal (BMJ), unlike the majority of the main stream press, is once again doing a great job at actually investigating and reporting on important issues. Yesterday’s investigation by Maryanne Demasi looked at conflicts of interest between big pharma and drug regulators.
Many doctors and the majority of the general public think that regulators provide unbiased assessments of future drugs and medicines but is this really the case? Over the last few decades more and more funding for regulators comes from the pharmaceutical industry. Maryanne asks the important question - “do they have sufficient independence from the companies they are meant to regulate?
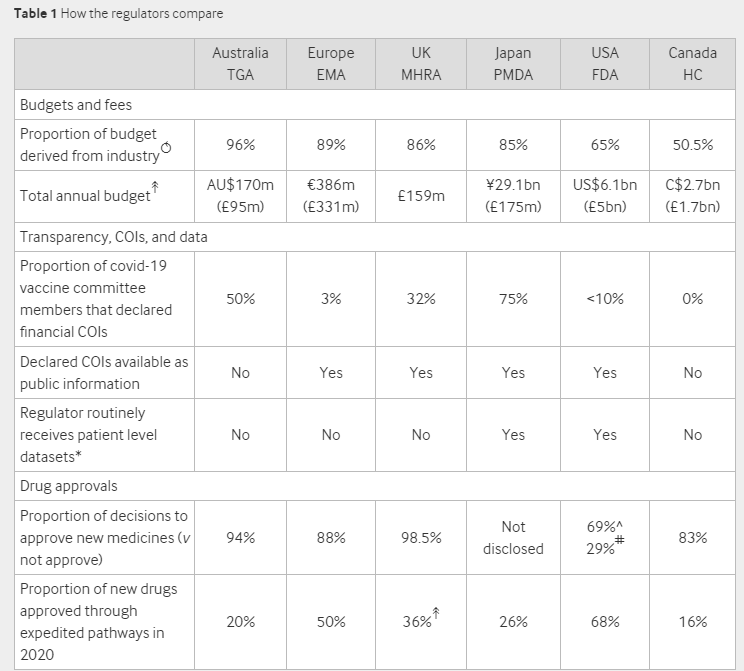
The BMJ questioned regulators from Australia, Canada, Europe, Japan, the UK and the US finding that “industry money permeates the globe’s leading regulators, raising questions about their independence, especially in the wake of drug and device scandals”.
Industry fees
All the regulators receive the majority of their budget from industry. A staggering 96% of Australia’s regulatory budget comes from industry with Canada receiving the smallest proportion at 50.5%.
Other interesting figures include a 98.5% approval rate of new medicines in the UK and 68% of new drugs approved through an expedited pathway in the US.
External advisers
The conflicts of interest aren’t just with the regulators themselves but also with the advisory panels they bring in to provide independent expert advice.
A BMJ investigation last year found several expert advisers for covid-19 vaccine advisory committees in the UK and US had financial ties with vaccine manufacturers—ties the regulators judged as acceptable. A large study that investigated the impact of COIs among FDA advisory committee members over years found that those with financial interests solely in the sponsoring firm were more likely to vote in favour of the sponsor’s product, and that people who served on advisory boards solely for the sponsor were significantly more likely to vote in favour of the sponsor’s product.
Transparency
Assessments of patient data is not usually undertaken by the regulators. Instead they use summaries provided by the pharmaceutical companies. And, as we have seen with the Pfizer documents, these data are not routinely published in the public domain.
The TGA [Australia’s Therapeutic Goods Administration], for example, says it conducts its covid-19 vaccine assessments based on “the information provided by the vaccine’s sponsor.” According to a FOI request from last May, the TGA said it had not seen the source data from the covid-19 vaccine trials. Rather, the agency evaluated the manufacturer’s “aggregate or pooled data.” The TGA does not have the individual participant level datasets pertaining to the covid-19 vaccine trials, which are held by the vaccine manufacturer.
Speedy approvals
All of the regulators questioned in the survey offered expedited pathways for new drugs. This has resulted in many new drugs which were more likely to be withdrawn for safety reasons.
“One reason why drugs approved by the FDA so close to the deadline may have had more safety problems is that the FDA reviewers were afraid of going over the deadline for making a decision and thereby jeopardising the revenue that the FDA gets from drug companies”.
Revolving doors
Funding is not the only method of regulatory capture. Many regulators end up working for the companies they had previously been regulating. Nine out of ten of the FDA’s past commissioners between 2006 and 2019 moved on to pharmaceutical companies.
Ian Hudson, chief executive of the UK’s MHRA between 2013 and 2019, now serves on the board of biotech company Sensyne Health and is a senior adviser for the Bill and Melinda Gates Foundation. Before joining the MHRA, Hudson held various senior roles at pharmaceutical giant SmithKline Beecham.
On a similar note, Sonia Elijah, writing at Trial Site News recently obtain leaked emails from the European Medicines Agency (EMA). These emails show many of the problems highlighted above.
An email from the former EMA Head of Biological Health Threats and Vaccines Strategy, predicted that the US Food and Drug Administration (FDA) were “going to rush into EUA” [Emergency Use Authorisation]. This was because they were being “pushed hard by Azar [former US Secretary of Health and Human Services] and US GOV Marco.
Another email from the EMA detailed how a teleconference with the European Commissioner, Ursula von der Leyen, was “rather tense, at times even a bit unpleasant, and provides a hint on what EMA may expect if the expectations are not being met, irrespective if such expectations are realistic or not”. The email continues “the political fall-out seems to be too high even if the “technical” level at the MSs [Member States] could defend such a delay in order to make the outcome of the scientific review as robust as possible”.
In late November 2020 an email from an EMA scientific administrator warned of issues between different batches of the Pfizer vaccines. There were significant differences in the percentage of RNA integrity meaning “the potential implication of this RNA integrity loss in commercial batches compared to clinical ones in terms of both safety and efficacy are yet to be defined.”
At around the same time, another EMA employee sent an update from the FDA about the Pfizer vaccines. Apparently if was “unclear if GCP [Good Clinical Practise] ever done (TBC), but no major interest from FDA".
To read these email and more go and read the whole article at TS News.
And finally for today, a doctor from the UK who was being disciplined for his views over Covid vaccines sent a letter to the disciplinary body, describing the reasons for his stance. His interesting letter can be read below.


















"Assessments of patient data is not usually undertaken by the regulators. Instead they use summaries provided by the pharmaceutical companies."
That is just bananas
Great post, great letter, thanks for including the citations. 💕👍🏽